
The human body heavily relies on protein molecules for proper functioning. Protein molecules are responsible for a host of biological tasks in the body from providing structural support of cells and tissues to the intricate process of detecting light and producing vision.
To execute their roles effectively, proteins undergo a precise folding process, transitioning them from a flexible chain of amino acids into their definitive three-dimensional configuration.
However, a significant number of proteins are unable to fold on their own and require the help of molecules called chaperones. When proteins are left without the guidance of chaperones, they often misfold or, even worse, aggregate with nearby substances and form clusters that can harm cellular integrity.
Thanks to new research from scientists at BYU and the University of Utah Health, researchers now have a more complete understanding of how chaperone molecules direct the protein folding process. The findings, recently published in Molecular Cell, challenge the existing model and offer potential pathways for addressing and rectifying folding issues when they occur.
To better understand the protein folding process that takes place within a cell, Barry Willardson, BYU professor of biochemistry, and Peter Shen, professor of biochemistry at the University of Utah Health, isolated a protein called Gβ5, which is involved in the responses of cells to hormones, neurotransmitters and sensory stimuli.
Leveraging an innovative microscopy process called cryo-electron microscopy, they isolated samples of Gβ5 and its chaperone, CCT, in the middle of the folding process. Willardson and Shen froze the Gb5-CCT complex and then placed it in an electron microscope to get a closer look. Images were captured from multiple angles and merged to create a 3D reconstruction of the complex.
The images surprised and excited the scientists. They discovered that CCT plays a much more active role in guiding the folding process of the proteins; disproving the prevailing theory that CCT acted more like a cage to protect the space for folding proteins.
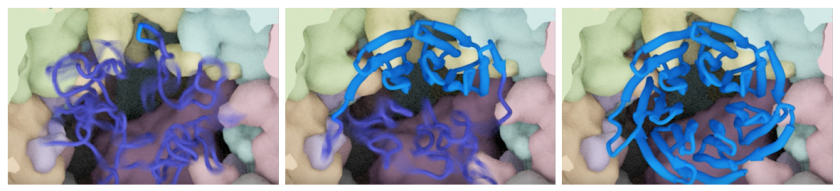
“For the first time we were able to visualize the complete folding trajectory of a protein sub-unit,” noted Willardson. “This is something scientists have been trying to visualize since the 1960’s and the 1970’s. As soon as we saw the images, we all knew that we had something unique.”
It’s a discovery that’s been in the making for more than five years, when students in Willardson’s lab began attempting to isolate samples of Gβ5 and its chaperone. The researchers say the discovery is an important step in treating diseases associated with mutations in Gβ units that interfere with folding and cause serious issues involving seizures and cognitive disability. Studying how the mutations affect the cells' responses to neurotransmitters will offer insights that will pave the way for future treatments of diseases.
“Now that we understand how the normal protein folds, we can apply the same tools and strategies to understand why the mutant protein does not fold properly,” said Shen in a news release posted by the University of Utah Health. “This gives us a much more informed way of trying to fix this problem at a molecular level.”
By Tyler Stahle, November 28, 2023
Media Contact: Tyler Stahle